no2- lewis structure
This problem has been solved. In the Lewis structure for NO2 the Nitrogen atom is the least electronegative atom and goes at the center of the structure.
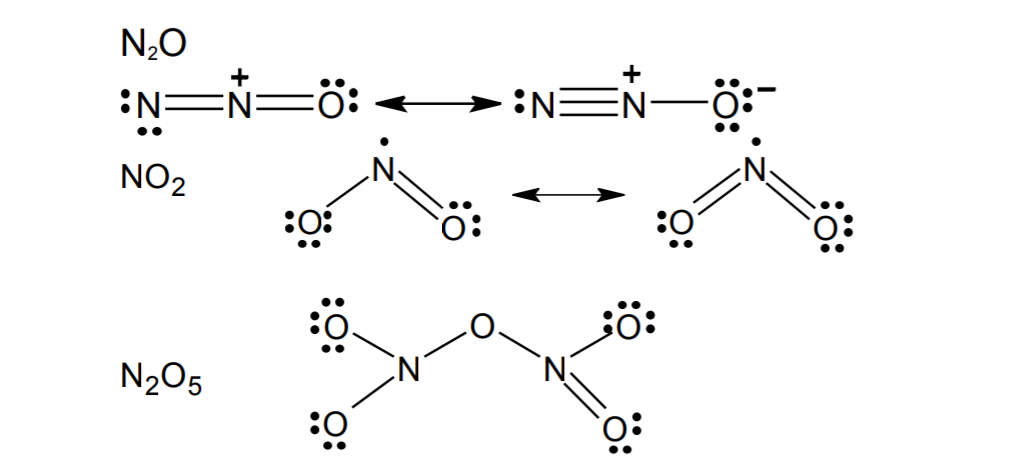 |
| Welcome To Chem Zipper Com Write The Lewis Structures Of N2o No2 And N2o5 |
You know that Nitrogen N has 5.
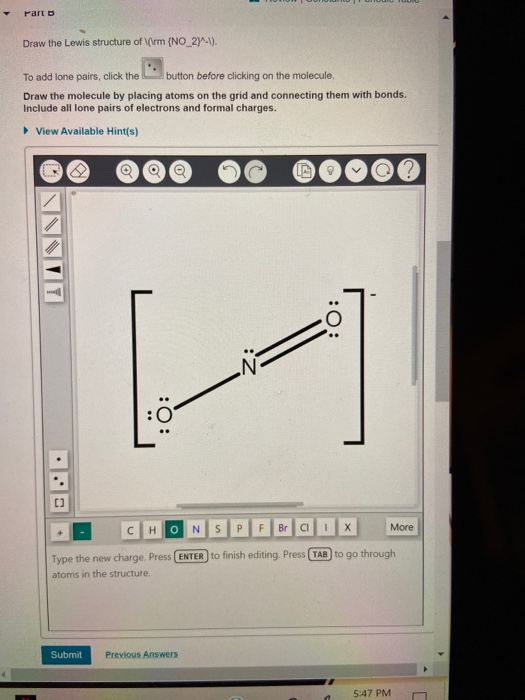
. Heres how you can draw the NO 2 lewis structure step by step. The left oxygen atom has two lone pairs the right. Draw the Lewis structure of NO2. Drawing the Lewis Structure for NO 2-Nitrite Ion.
It is a weak acid and exists only in specific conditions ie in solution cold and dilute as a gas or in the form of nitrite salts. Talking about its properties SO2 has a molar mass of 64066. Please find the Lewis Dot Structure for NO2 also known as nitronium ion shown above. Now in the above sketch of NS2 molecule put the two electrons ie electron pair between each.
You can learn basics of how to draw a lewis. Lewis structure of NO 2 Nitrogen dioxide is drawn in this tutorial step by step. This is a pungent-smelling colorless gas. A step-by-step explanation of how to draw the NO2 - Lewis Dot Structure Nitrite ionFor the NO2 - structure use the periodic table to find the total number.
Now in the NO2 molecule you have to put the electron pairs between the nitrogen atom N and oxygen atoms O. NO2 Lewis structure bond angle. NO2 lewis structure explain with this techniques First of all you find the number of balance electron of NO2 ion. For the NO2 Lewis structure calculate the total number of valence.
For the NO2- Lewis structure calculate the total number of valence electrons for the NO2- molecule. As with all other Lewis Dot Structures the bonds within the structure can be replaced by. Youll get a detailed solution from a subject matter expert that helps you learn core concepts. HNO2 is also known as Dioxonitric III acid.
After determining how many valence electrons there are in NO2- place them around. NO2 lewis structure. There are two bonded pairs and an unpaired electron in the Lewis structure for NO2. When we examine the nitrate ion NO2- we notice that it contains two bond pairs one lone pair.
Bond angle is another physical feature identified by the Lewis structure of any compound. It is also known as an alkali metal oxide as it comprises two sodium. Put two electrons between the atoms to represent a chemical bond. Nitrogen dioxide is a highly reactive chemical compound with a molecular formula of NO2.
Bond angle also can be obtained with the help of VSEPR Valence. Heres how you can draw the lewis structure of NO 2 step by step. The Lewis structure for NO 2-Nitrite Ion comes up quite often in chemistry. Na2O Lewis Structure Uses and Properties.
Lewis Structure for NO 2 Nitrogen dioxide Oxidation number. Be sure to put brackets along with a negative. A step-by-step explanation of how to draw the NO Lewis Dot Structure Nitronium ionFor the NO structure use the periodic table to find the total number o. The lewis structure of NO2 contains one double bond and one single bond with nitrogen in the center and two oxygens on either side.
Sulfur dioxide is spelled as Sulphur dioxide in Commonwealth English. Nitrogen dioxide is liquid at room temperature and converts to yellow or reddish-brown at. This indicates that the nitrogen N and oxygen O are chemically bonded. Sodium Oxide having a chemical formula of Na2O is a metal oxide.
 |
| No2 Lewis Structure Nitronium Ion Youtube |
 |
| Solved 31 Question 3 Points Use The Knowledge And Chegg Com |
 |
| Re What Is The Proper Lewis Structure For No2 And Its Properties |
 |
| No2 Nores Png |
 |
| Explain Why Does No2 Dimerise Curlyarrows Chemistry Tutorials |
Posting Komentar untuk "no2- lewis structure"